Jodas is a Global, specialty, innovation driven, emerging, generic pharmaceutical company, that is asserting itself amongst the world’s foremost pharmaceutical companies. We began our journey of growth in the year 2008 in India, and today Jodas is a modern, research driven organization that has quality and affordability at the heart of its actions.
Jodas focuses its operations on four specialty therapeutic segments:
- Anti Infectives (Injectables)
- Anti Cancer (Injectables & Orals)
- Contrast Media (Injectables)
- Critical Care (Injectables)
Our dedicated team of healthcare professionals strives to bring speciality, high-quality, effective and affordable generic formulations to the market, touching millions of lives with good health. Jodas has approved products in 50+ countries with State-of-the-art world-class manufacturing facilities and has successfully commercialized 19.4 million Injectables in the year 2022, with unparalleled customer satisfaction. The future endeavour is to be a Global generic player, validating our commitment to “ONE STEP BETTER”, to fulfil the unmet medical needs of the healthcare community around the Globe with speciality generic formulations of uncompromising quality. Jodas has constantly raised the bar in maintaining uncompromising quality, with a pulse on rapidly changing innovation to the commercialization landscape through our selection of a product range of affordable medicines and speed-to-the-market, along with our partners and Global supply chain. We continuously evolve our business focus with a customer–centred portfolio of niche and technologically challenging products from speciality and therapeutic segments. Our continuous focus on innovation and quality drives us to fulfil the unmet medical needs of the healthcare community and make the dreams of millions of people across the world come true with much-needed medicines at affordable value.

Our centre of operations is headquartered in Hyderabad, India. Our world-class, state-of-the-art manufacturing facility and Innovation Center is located at Genome Valley – India’s first-of-its-kind cluster SEZ, designed as a hub for pharmaceutical biotech companies.
Total Plot Size: 72000 Sq.m
Built-up area: 33000 Sq.m
No of manufacturing Lines: 7 Lines
Labs: Sophisticated F R&D, A R&D, Quality control and Microbiology Labs
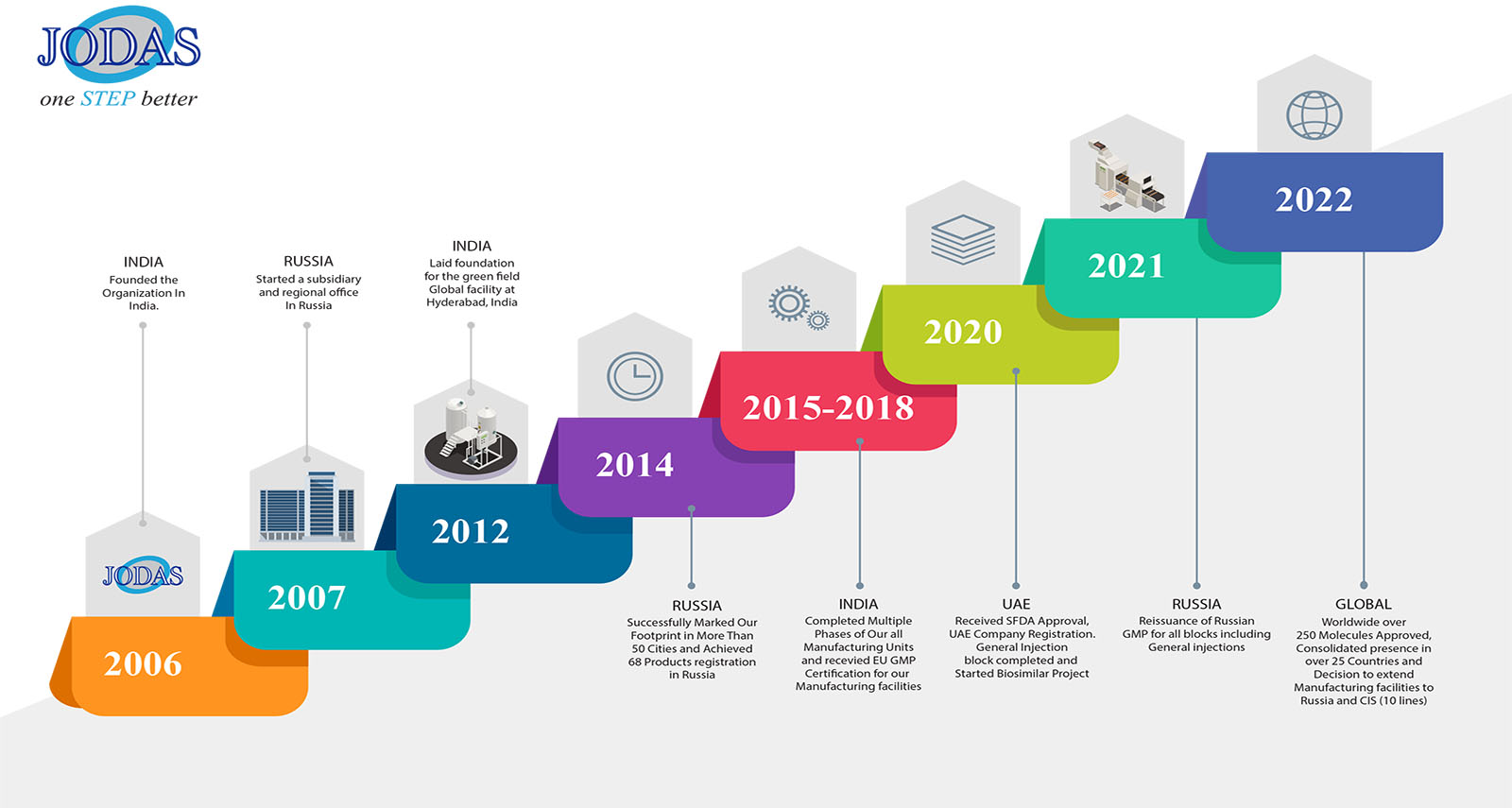
Jodas has evolved significantly, as a generics new generation pharmaceutical company-
2006
- Founded the organization in India.
2007
- Started an office in Russia.
- 3 molecules of anti-infectives commercialized in Russia.
2010
- 14 molecules of anti-infectives commercialized in Russia.
- Launched Oncology products in Russia.
2012
- Achieved 38 SKUs commercialization in Russia.
- Laid foundation for the green field Global facility at Hyderabad, India.
2013
- Launched Cardiac Critical Care and Radio Contrast Media products.
2014
- Successfully marked our footprint in more than 50 cities in Russia.
- Achieved 68 Products registration in Russia.
2015
- Completed Phase –I (Cephalosporin’s unit) of our Global facility in Hyderabad, India.
2016
- Completed Phase –II Oncology block for orals (Oncology orals) of our Global facility in Hyderabad, India.
2017
- Completed Contrast media unit of our Global facility in Hyderabad, India.
2018
- Completed Oncology sterile unit of our Global facility in Hyderabad, India.
- EU GMP certification of Cephalosporin, Oncology (Oral/Solid dosage and Injection blocks), Contrast Media (SVP+ LVP + PFS) Block.
- Filing of many registrations in EU+ Russia+ UK+ Saudi and ROW.
2019
- Approvals for many drugs in the EU and Russia.
- Started conducting Clinical trials and BE in all above said countries.
2020
- SFDA approval, UAE company registration.
- Many approvals/ filings in EU, UK, Russia, Saudi, and ROW.
- General Injection block completed.
- Started Biosimilar project.
2021
- Approvals from UAE, Saudi, Russia, and EU.
- CT and BE completed on many drugs worldwide.
- First Biosimilar drug Pertuzumab in PCT stage.
- Reissuance of Russian GMP for all blocks including General injections.
2022
- Worldwide over 250 molecules Registrations (Approvals).
- Worldwide over 200 molecules are under registration.
- Consolidated presence in over 25 countries.
- Second Biosimilar drug (pembrolizumab) initiated.
- Decision to extend manufacturing facilities to Russia and CIS (10 lines).
- New project with three facilities (to be constructed) in Hyderabad.
- Jodas approved by 9 Regulatory bodies including the EU, Russia, PIC/s, UAE & SFDA.
At Jodas, we ensure that our promise of caring for our consumers, our commitment to our employees’ growth and welfare, our continuous quality focus, and the spirit of innovation that drives each of us to discover better ways of working stay un-affected.
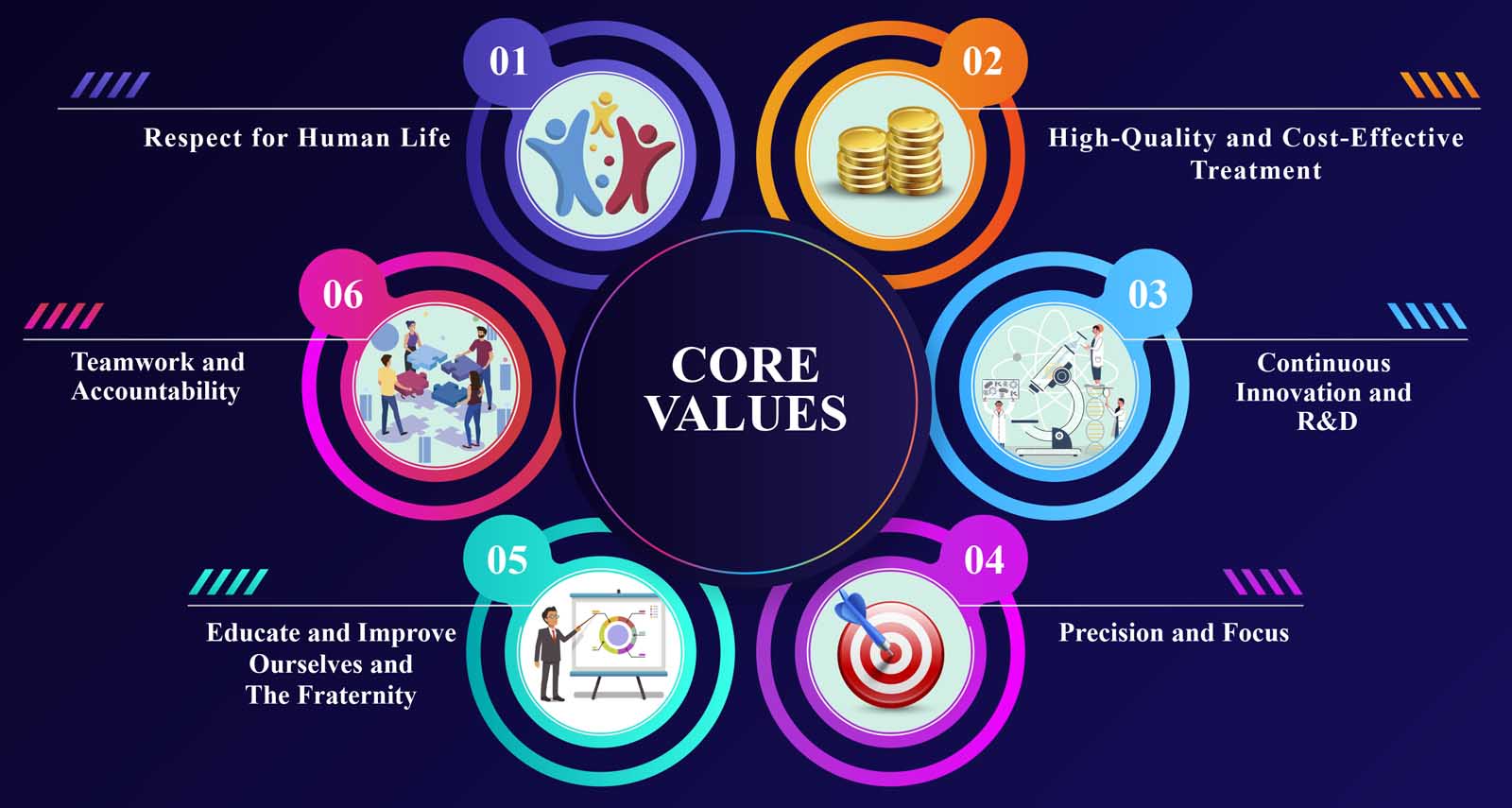
Core Values that define us
- Respect for Human Life.
- Determination to give high-quality and cost-effective treatment.
- Continuous Innovation and R&D.
- Precision and Focus.
- Educate and improve ourselves and the fraternity.
- Teamwork and Accountability.
Regulatory Approvals
Our Presence