TRANSFORMING LIFE TOWARDS BETTER LIVING
Jodas Expoim is a well-integrated research-driven biopharmaceutical manufacturing company, developing intellectual property through technology acquisitions, collaborative networking, and vibrant in-house R&D programs, also has robust research and development pipeline for biosimilars with platform-driven technology and leading manufacturing capabilities to manufacture and commercialize innovative products related mainly to immuno-oncology and autoimmune-related disorders. Our Bio-technical department consist of a young, dynamic multidisciplinary team with Biotechnology, Microbiology, Biochemistry, and Bio-chemical engineering background with expertise in Biosimilars development, manufacturing, and commercialization backed by visionary leadership. We intend to develop effective biosimilars monoclonal antibodies and other recombinant biotherapeutics to meet the utmost medical needs of the common man.
Established in Biotech Park Phase III in Hyderabad, India, in 2020, Jodas Expoim is committed to providing high-quality biologics that are accessible and cost-effective for patients globally. Spread over 20,000 SQM, our state-of-the-art facility possesses advanced capabilities such as development, manufacturing, pre-clinical and clinical programs for biotech products. A cGMP facility for cell culture-based biotherapeutics, both Drug Substances and Drug Products.
The State of the Art Biotech Facility :
R&D Initiatives
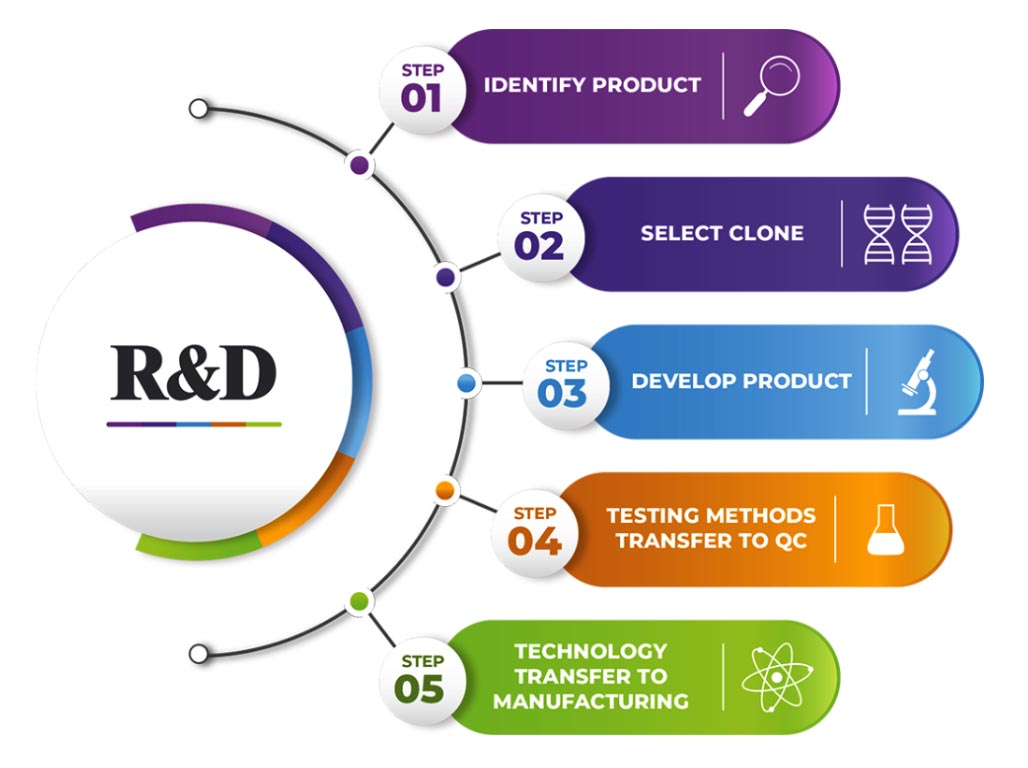
- The state-of-the-art R&D Centre is dedicated to develop novel, effective and affordable Biopharmaceuticals for the prevention and cure of life-threatening diseases for India and global markets.
- R&D Centre has the infrastructure to carry out extensive research in biotherapeutics using genetic engineering, molecular biology, genomics tools, animal cell culture, fermentation, purification, formulation, serology, and analytical testing techniques.
- R&D Centre has the infrastructure and expertise to take an idea through different stages of product development towards successful commercialization.
- R&D Centre’s focus is on the development of preventive and therapeutic biopharmaceuticals, novel therapeutic proteins, growth factors, hormones, and therapeutic monoclonal antibodies for treating infectious diseases and lifestyle-related disorders.
- The R&D center located at Biotech Park Phase III in Hyderabad, India is spread over 500 square meters of area and equipped with all modern equipment for the process development of recombinant biotherapeutics.
R&D Infrastructure
The R&D center having well classified laboratories to handle different stages of development of Biotherapeutics –
- Molecular Biology lab
- Upstream process development and scale up lab
- Downstream process development and scale up lab
- Analytical method development lab
- Bio Assay development lab
Major Equipment available to support the process development, optimization, scale up and validation
- Eppendorf Bioreactor
- AKTA Pure and AKTA Pilot Chromatography systems
- TFF systems
- Lyophilizer
- Waters HPLC with UV & FL detectors and UPLC
- Biorad RT-PCR
- Biorad Electrophoresis and Western blot apparatus
- Stability chambers
- Multimode Spectrophotometer with fluorescence detector
- Biosafety Cabinet (BSL II)
- Bio profile analyzer
- CE-SDS &CE-IEF
- UV-Visible spectrophotometer

R&D Capabilities
- Clone development with proven platform technology.
- Process development from R&D to Pilot scale
- Batch, fed-batch, and perfusion strategies for biosimilars products
- Downstream Process development with modern chromatography systems, Ultrafiltration-Diafiltration systems and Lyophilizer
- Process and Product characterization
- Process Scale-up and tech transfer to cGMP manufacturing plant
- Formulation development
- Analytical method development, qualification and validation
- Bioassays development qualification and validation
- Drug Substance and drug product stability studies
- Product characterization
Our pipeline of biosimilars
- Main focus is on Cytokines, Growth Factors and therapeutic Monoclonal Antibodies.
- Our robust pipeline of 7 biosimilars (products produced by recombinant DNA-technology in Chinese Hamster Ovarian cell lines) are at various stages of development, from process development to pre-Clinical stage. However, the following three are at advanced stages of development-
1. Pertuzumab – For treatment of Breast Cancer
2. Pembrolizumab and Nivolumab – PD-1 antagonists.
3. Atezolizumab – PD-L1 antagonists.
Our Manufacturing Capabilities
- Manufacturing of cell culture based biotherapeutics under cGMP conditions, both Drug Substances and Drug Products
- The manufacturing facility is built to meet the EU-GMP and USFDA regulatory requirements
- The manufacturing facility is spread over 1,800 SQM and located at Biotech Park Phase III in Hyderabad, India
- Dedicated State of the art cGMP facility with all modern equipment to run the manufacturing process of biotherapeutics in a seamless manner
- The finished product in pre-filled Syringes and Vials
- State of the art QC lab for release test of drug substances and drug products
- Superior cold chain system for maximum care in storage and supply of products
- Well establish Quality Management System (QMS) at various levels
Manufacturing Infrastructure
- Bioreactor – Trains of 10L, 100L, 500L and 1000L for seed preparation to production for clinical trial requirement and commercial manufacturing of bulk drugs
- Integrated Downstream set up with modern chromatography systems, depth Filtration unit, TFF and lyophilizer for purification and production of drug substances
- Fill-Finish Facility for liquid formulation in Pre-filled Syringes and Vials
- Fill-Finish Facility for Lyophilized formulation in Vials
- Dedicated utilities: HVAC, Water systems-PW, WFI, PSG and compressed air, etc.
- In process testing Labs and Warehouse



